Product Approval Progress
In the first quarter of 2021, 3 pharmaceutical excipients of our company turned into “A(active)” status through joint review with preparations. Our company newly registered 6 pharmaceutical excipients and 2 APIs that were already publicized on the CDE platform.
Pharmaceutical Excipients
1.Polysorbate 80(Tween 80 HP)
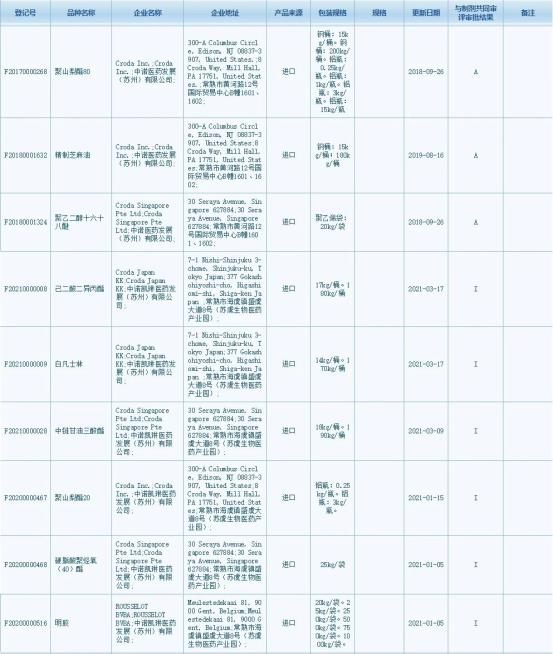
Polysorbate 80 was approved by CDE on February 1st and changed into “A” status with the registration number of F20170000268
· Origin: US
· Characteristics: In accordance with USP, EP, JP and ChP, low moisture and low peroxide value.
· Application: commonly used as O / W emulsifier, solubilizer and humectant
2.Sesame Oil SR
Sesame oil SR was approved by CDE on February 1st and changed into “A” status with the registration number of F20180001632
· Origin: US
· Characteristics: In accordance with USP, EP, JP and ChP. Croda adopted its unique super refined technology to purify sesame oil by removing polar impurities (such as primary and secondary oxidation products) in natural oil. Croda improves the color, odour and smell of the product without changing the form of basic triglyceride. It increases the stability of the preparation by reducing the adverse impact on APIs.
· Application: Sesame oil is an ideal solvent for oil-soluble sustained release injections like steroid, avermectin and etc. It can also be used in suspension, emulsion, ointment, ophthalmic preparation and suppository.
3.Macrogol Cetostearyl Ether
Macrogol Cetostearyl Ether was approved by CDE on January 7th and changed into “A” status with the registration number of F20180001324.
· Origin: Singapore
· Characteristics: In accordance with USP, EP
· Application: Macrogol Cetostearyl Ether is used as emulsifier in cream and emulsion. It is also used as solubilizer in essential oils, vitamin oils and low water-soluble drugs. It is relatively stable under moderate and strong acid and alkali conditions.
4.Diisopropyl Adipate
Diisopropyl Adipate was publicized on the CDE platform on March 17th with the status of “I” and its registration number is F2021000008
· Origin: Japan
· Characteristics: In accordance with JP; extremely low viscosity; good solubility and ductility.
· Application: It is recommended to be used as solvent or carrier for external preparation.
5.White Vaseline
White Vaseline was publicized on the CDE platform on March 17th with the status of “I” and its registration number is F2021000009
· Origin: Japane
· Characteristics: In accordance with JP, EP, JP, ChP. Croda adopted its unique super refined technology to remove the polar impurities in Vaseline, thus enhancing the stability of the preparation and reducing the potential skin irritation.
· Application: It is mainly used as moisturizing ointment base for topical administration drugs.
6.Medium-Chain Triglycerides
Medium-Chain Triglycerides was publicized on CDE platform on March 9th with the status of “I” and its registration number is F20210000028
· Origin: Singapore
· Characteristics: In accordance with USP, EP, ChP; good oxidation stability
· Application: It can be used as solubilizer and carrier of oil-soluble active substances, filler and lubricant of capsules and tablets, as well as medium of anti-parasite pour-on solution. In addition, it can also be used as good emulsifier due to its excellent oxidation stability.
7.Polysorbate 20
Polysorbate 20 was publicized on CDE platform on on January 15 with the status of “I” and its registration number is F20200000467
· Origin: US
· Characteristics: In accordance with ChP, USP, EP; low moisture and low peroxide value.
· Application: It is commonly used as O / W emulsifier, solubilizer and humectant.
8.Polyoxyl (40) Stearate
Polyoxyl (40) Stearate was publicized on the CDE platform on January 5th with the status of “I” and its registration number is F20200000468
· Origin: Singapore
· Characteristics: In accordance with USP, EP and CHP; it is more stable, transparent and has better quality when combines with cetostearyl alcohol , stearic acid, glycerin or sorbitol monoester / diester
· Application: It is a kind of non-ionic surfactant, mainly used as emulsifier and solubilizer for preparing emulsions and creams.
9.Gelatin
Gelatin was publicized on the CDE platform on January 5 with the status of “I” and its registration number is F20200000516
· Origin: Belgium (Rousselot)
· Characteristics: In accordance with CHP, EP and USP. It is animal derived protein extracted from pigskin collagen with good congelation /film-forming ability, adhesion, stability, plasticity and / or disintegration characteristics.
· Application: it can be used in the production of hard capsule, soft capsule, tablet, coating agent and microcapsule.
APIs

1.Prilocaine
Prilocaine was publicized on CDE platform on March 12 with status of “I” and its registration number is Y20200001320
· Origin: India (Gufic Biosciences Ltd)
· Specification: In accordance with EP
· Application: it is used as amide topical anesthetic in cream.
2.Cilastatin Sodium
Cilastatin Sodium was publicized on the CDE platform on March 25 with the status of “I” and its registration number is Y20200001273(Sino Caring is the exclusive agent of Cilastatin Sodium in China)
· Origin: Italy (ACS Dobfar S.P.A.)
· Specification: In accordance with EP
· Application: It is renal peptidase inhibitor that combines with carbapenem antibiotic imipenem. It is broad-spectrum antibacterial for the treatment of various moderate and severe infections.
Sino Caring follows the philosophy of “Pursue Harmony, Honor Commitment "and practices the value of “high quality, innovation, conscientiousness, sharing”. Sino Caring keeps improving itself to become the first-class service-oriented company in pharmaceutical industry!


